See this Doctor Discussion Guide to find out about topics your patients may raise with you regarding their migraine experiences and treatment goals.

Why Nurtec® ODT (rimegepant)
Jennifer F.
Actual Nurtec ODT patient.
*Per IQVIA as oral brand in class (oral CGRP receptor antagonists): number one prescribed and number one in new prescriptions, since 8/6/21. Data current as of 8/21/23.
Patients are often forced to sit on the sidelines, unable to participate in the things they love1
In a survey of 11,266 people with migraine…2,†
59% said that migraine has stopped them from participating in previous hobbies2,†
57% said that migraine has stopped them from attending social events2,†
34% said that migraine has stopped them from engaging in sports or exercising2,†
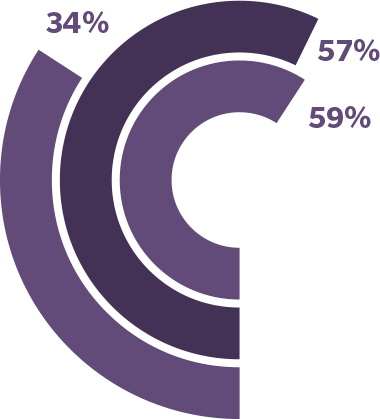
†The My Migraine Voice global study was a 2018 online survey to assess disease burden. Eligible participants included 11,266 adults with migraine who reported ≥4 monthly migraine days within the 3 months preceding recruitment.2

I'm so glad that Nurtec ODT
can do so much for my
migraine attacks!
Evan B.
Actual Nurtec ODT patient.
Individual results may vary.
It was like winning back something I had given up. Nurtec ODT worked for me!
Mary H.
Actual Nurtec ODT patient.
Individual results may vary.
Patients were invited to share their experiences on Nurtec ODT. All content was accurate at the time of publication.
I have had migraines for 30 years. I am so happy my doctor told me about Nurtec ODT.
Natalie R.
Actual Nurtec ODT patient.
Individual results may vary.
When a migraine strikes, I'm so happy to have Nurtec ODT to get me through my day.
Tomeka C.
Actual Nurtec ODT patient.
Individual results may vary.
Patients were invited to share their experiences on Nurtec ODT. All content was accurate at the time of publication.
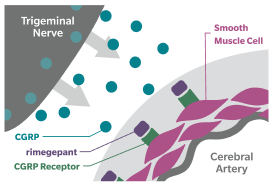
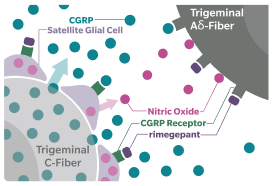
Blocking the CGRP receptor has been shown to reduce migraine pain, symptoms, and frequency6,7
CGRP=calcitonin gene-related peptide; MOH=medication overuse headache.
*The safety of using more than 18 doses in a 30-day period has not been established.4
Nurtec ODT has helped with my migraine pain immensely!
Linda R.
Actual Nurtec ODT patient.
Individual results may vary.
The medication I trust to stop migraine attacks is proven to help prevent migraines, too? That's amazing!
Brittany N.
Actual Nurtec ODT patient.
Individual results may vary.
Patients were invited to share their experiences on Nurtec ODT. All content was accurate at the time of publication.
References: 1. Migraine in America 2020 Findings for Media. Health Union, LLC. 2. Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018 Nov 27;19(1):115. doi:10.1186/ s10194-018-0946-z. 3. Health Union, LLC. Migraine in America 2021. Health Union, LLC; 2020. 4. Nurtec ODT. Package insert. Pfizer Inc. 5. Ishii R, Schwedt TJ, Trivedi M, et al. Mild traumatic brain injury affects the features of Migraine. J Headache Pain. 2021;22(1). doi:10.1186/s10194-021-01291-x 6. Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(Suppl 1):99-105. doi:10.1007/s10072-019-03769-8 7. Dubowchik GM, Conway CM, Xin AW. Blocking the CGRP pathway for acute and preventive treatment of migraine: the evolution of success. J Med Chem. 2020;63(13):6600-6623. doi:10.1021/acs.jmedchem.9b01810 8. Durham PL. CGRP‑Receptor Antagonists — A fresh approach to migraine therapy? N Engl J Med. 2004;350(11):1073-1075. doi: 10.1056/NEJMp048016 9. Ailani J, Burch RC, Robbins MS; the Board of Directors of the American Headache Society. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache. 2021;61:102-1039. doi: 10.1111/head.14153